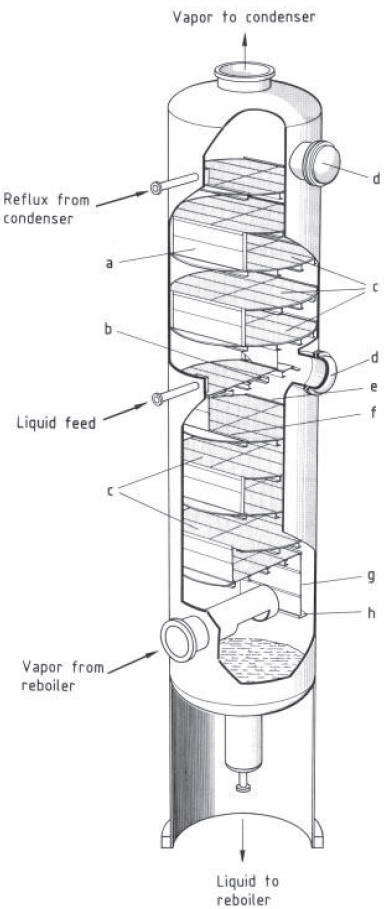
Cutaway section of a tray column
a) Downcomer;
b) Tray support;
c) Sieve trays;
d) Manway;
e) Outlet weir;
f) Inlet weir;
g) Side wall of downcomer;
h) Liquid seal [1]
Distillation successful because fluids are handled easily
…only fluid phases, which can be handled very easily, are involved.
A further advantage is a high density difference between the coexisting phases. High density differences enable high velocities in the equipment and make the separation of the phases easier.
Distillation successful because energy can be used efficiently
Disadvantages of distillation and rectification are a risk of thermal degradation of substances and, even more importantly, a high energy demand.
This high energy demand, however, can be drastically reduced by special measures, e.g., by material and thermal coupling of the equipment, by implementing heat pumps or by complete heat integration of the process.[2] …this energy frequently can be in the form of exhaust steam from turbine drives or other waste heat that has a relatively low value. In fact, reboilers may provide a utility service by using heat that otherwise might be wasted.[3] …the theoretical work can be closely approached by actual work after known inefficiencies are identified and… the dominant driving force losses are in pressure drop and temperature difference.[4]
Process data and column internals are highly developed
Rectification [distillation] is a highly developed technology with respect to thermodynamic fundamentals (e.g., vapor–liquid equilibrium, thermodynamics) as well as design and construction of the equipment. Rectification columns can be safely constructed and operated up to diameters of 10 m and heights of 100 m.
Column internals (e.g., trays, packings) are very effective in enhancing the interfacial mass transfer…
All these advantages often make rectification the separation technology of choice. Whenever a fluid mixture can be fractionated [separated into components] by rectification then rectification is in most cases the winner of a comparison of several separation techniques…[2]
- Stichlmair, Johann. “Distillation and rectification.” Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, p. 74.
- Mersmann, Alfons, Matthias Kind, and Johann Stichlmair. Thermal separation technology: principles, methods, process design. Springer Science & Business Media, 2011, p. 231.
- Kunesh, J. G., et al. “Distillation-Still Towering over Other Options.” Chemical Engineering Progress 91.10 (1995): 43-54.
- Steinmeyer, Dan. “Process Energy Conservation.” Kirk-Othmer Encyclopedia of Chemical Technology. 5th ed., Wiley-Interscience, 2004-2007, p. 6.